Abstract
Background: Ibrutinib is the first-in-class Bruton's tyrosine kinase inhibitor (BTK) approved by the FDA as breakthrough therapy for the treatment of B cell lymphomas and CLL at 560 mg or 420 mg once daily. However, the inter-pt pharmacokinetic (PK) exposures vary significantly which may impact both efficacy and toxicity. A novel BTK inhibitor which achieves target drug exposures in all relevant populations (even at low doses) may provide consistent efficacy across pts with a favorable, dose dependent side effects profile. In addition, such a differentiated BTK inhibitor may serve as a backbone for combination therapies with small molecule and/or antibody drugs and/or cellular therapies. DTRMWXHS-12 (or DTRM-12) is a selective, irreversible and potent BTK inhibitor (IC50 0.7 nM). Off-target screening with 104 proteins (e.g., transporters, cell receptors, ion channels and enzymes) indicates no inhibitory activity. We investigated small molecule combinations for potential synthetic-lethal activity through screening and optimization studies and we are currently targeting multiple key signaling pathways via BTK and mTOR inhibition combined with an IMID to improve selective cell kill and avoid acquired drug-resistance (NCT02900716). Here we report the results of the Simultaneous Global Phase I Studies of a Differentiated BTK Inhibitor DTRMWXHS-12 monotherapy in relapsed/refractory (r/r) Patients with CLL and B-cell lymphomas.
Methods: China Trial (NCT02891590): A phase I, first-in-human trial evaluated escalating doses of DTRM-12 monotherapy from 50, 100, 200, to 400 mg daily to determine safety, anti-tumor activity and pharmacokinetics. Eligible pts are ≥18 years of age, with an ECOG ≤ 1 with r/r B-cell malignancies with no available therapies. Treatment was administered for 28 consecutive days out of of a 35-day cycle, until disease progression (PD) or unacceptable toxicity. The first pt was dosed on 11/2/2016.
US Trial (NCT02900716): A phase I, first-in-human multicenter trial exploring DTRM-12 as monotherapy or in combination in pts with r/r CLL, Hodgkin lymphoma and B-cell NHL. Phase Ia is reported and consists of escalating DTRM-12 monotherapy doses (50-200 mg). Safety of the mono- and combined therapies, anti-tumor activity and pharmacokinetic studies are being evaluated. Eligible pts are ≥18 years / ECOG ≤ 1 with r/r CLL or B-cell NHL with no available therapies. Treatment is administered for 21 consecutive days of a 28-day cycle, until disease progression or unacceptable toxicity. The first pt was dosed on 9/27/2016.
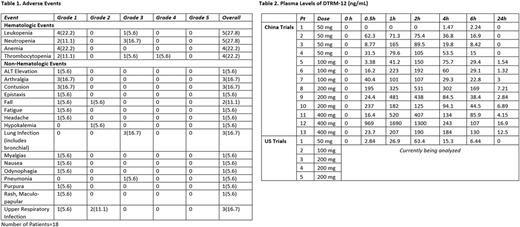
Results: Thirteen pts in China were enrolled (4 pts at 50mg; 3 pts at 100/200/400mg respectively) including 7 follicular lymphoma (FL), 3 Waldenstrom's macroglobulinemia (WM), 2 marginal zone, (MZL), and 1 CLL. Median age was 56 yrs (range 30-72); 69% male, 2 median prior treatments (range 1-6). No DLTs occurred and the MTD was not reached at 400 mg. In the China cohort, 1 pt withdrew consent on study day 3 (50 mg); 3 pts have discontinued due to PD and 9 remain on therapy. With a median follow up 4.7 months (1.6-8.6), no pt discontinued due to an adverse event (AE). Five pts in the US were treated with DTRM-12 (1 pt at 50 mg, 1 pt at 100 mg and 3 pts at 200 mg) including 3 B-cell lymphomas (2 DLBCL, 1 WM) and 2 CLL. Median age was 61.5 yrs (range 46-77); 60% male, median prior treatments 3 (range 1-4). No DLTs occurred and the MTD was not reached at 200 mg. In the US cohort, 3 pts remain on therapy, with a median follow up of 8.25 months, while 2 DLBCL pts discontinued for PD at 2 and 4 months. Table 1 describes all cause adverse events (AEs) for subjects across both studies (n=18). Available plasma levels of DTRM-12 on day 1 in both American and Chinese cohorts are presented in Table 2.
Conclusions: Results of two simultaneous phase I studies demonstrate that DTRM-12 monotherapy is well tolerated across B cell malignancies and CLL in 18 pts. In both studies, no DLTs occurred and an MTD was not identified. PK studies demonstrate adequate target drug exposures in all pts at all dose levels. Due to its favorable side effect profile, PK consistency across dose levels and pre-clinical data supporting the concept of synthetic lethality, DTRM-12 is currently being studied in combination with low doses everolimus and pomalidomide in advanced B cell lymphomas and CLL (protected by US patents US9532990 and US9717745).
Schuster: Bristol-Myers Squibb: Consultancy, Research Funding; Genentech: Consultancy, Research Funding; Janssen: Consultancy, Honoraria; Nordic Nanovector: Consultancy; Seattle Genetics: Consultancy; Novartis: Consultancy, Research Funding; Gilead: Consultancy, Research Funding; Celgene: Consultancy, Research Funding; Merck: Research Funding. He: DTRM biopharma: Employment; DTRM biopharma: Equity Ownership; DTRM biopharma: Other: leadership. Dwivedy Nasta: Incyte: Research Funding; Takeda: Research Funding; Immunogen: Research Funding. Landsburg: Curis: Consultancy, Research Funding; Takeda: Research Funding. Porter: Servier: Honoraria, Other: Travel reimbursement; Incyte: Honoraria; Novartis: Honoraria, Patents & Royalties, Research Funding; Immunovative Therapies: Other: Member DSMB; Genentech/Roche: Employment, Other: Family member employment, stock ownship - family member. Svoboda: Merck: Research Funding; Kite: Consultancy; BMS: Consultancy, Research Funding; Seattle Genetics: Consultancy, Research Funding; Celgene: Research Funding; Pharmacyclics: Research Funding. Mato: Celgene: Consultancy, Membership on an entity's Board of Directors or advisory committees; Kite: Consultancy; AbbVie: Consultancy, Research Funding; TG Therapeutics: Membership on an entity's Board of Directors or advisory committees, Research Funding; Pharmacyclics: Research Funding; Janssen: Consultancy; AstraZeneca: Consultancy; Acerta: Research Funding; Portola: Research Funding; Regeneron: Research Funding; Gilead Sciences, Inc.: Membership on an entity's Board of Directors or advisory committees; DTRM: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.